Probiotics: what's in a name?
IPA Europe brings together a forum of leading producers of probiotic cultures, probiotic foods, supplements, nutritionals and therapeutic products

Press Package

Better consumer information and a thriving probiotics industry in Europe
The IPA Europe Manifesto

IPA Europe and European Dairy Association (EDA) joint statement on probiotics
For a better regulatory environment for probiotic food and food supplements in Europe

The science behind probiotics
Discover the 4 criteria to qualify microorganisms as “Probiotic” in foods and dietary supplements
IPA Europe's priorities:
better information for consumers,
better rules for the industry,
better quality of life
IPA EUROPE
Call for a responsible use of the term 'probiotic'
READ THE STATEMENT
IPA Europe News
Engaging European Deputies: Seeking Support for Probiotic Improvement in Europe
IPA Europe is advocating for European policymakers to reassess the criteria governing the term ‘probiotic’ for food and food supplements within the EU. The current regulatory framework dates back to 2007, placing European stakeholders at a competitive disadvantage. Read our statement published in the April edition of ‘The Parliament Magazine’ ...
European industries call for a bold and impactful Biotechnology and Biomanufacturing Initiative
Brussels, 22 February 2024 Cross sectoral industries have come together to champion the European Commission’s forthcoming Biotechnology and Biomanufacturing Initiative through a position paper which calls for bold ambition, and five core principles to ensure impactful industrial growth. The ‘EC Initiative on Biotechnology and Biomanufacturing: Principles for an impactful Europe’ ...
IPA Europe Announces Dr. Bart Degeest as New President
IPA Europe, representing probiotic food and food supplements in Europe, is pleased to announce the election for the new President, Dr. Bart Degeest, during the Board meeting of the association held in Milan. Dr. Degeest has consistently demonstrated a profound understanding of the challenges andopportunities facing our community and contributes ...
IPAEU PRESS RELEASE: ROME CONGRESS 2023
Brussels, 11 September 2023 The International Probiotic Association (IPA & IPAEurope) is returning with the 3rd Science & Business Symposium during the 12th Probiotic, Prebiotics, and New Food Congress (Rome 16-19 September 2023) IPA with IPAEurope is returning with the 3rd Science & Business Symposium during the 12th Probiotic, Prebiotics, ...
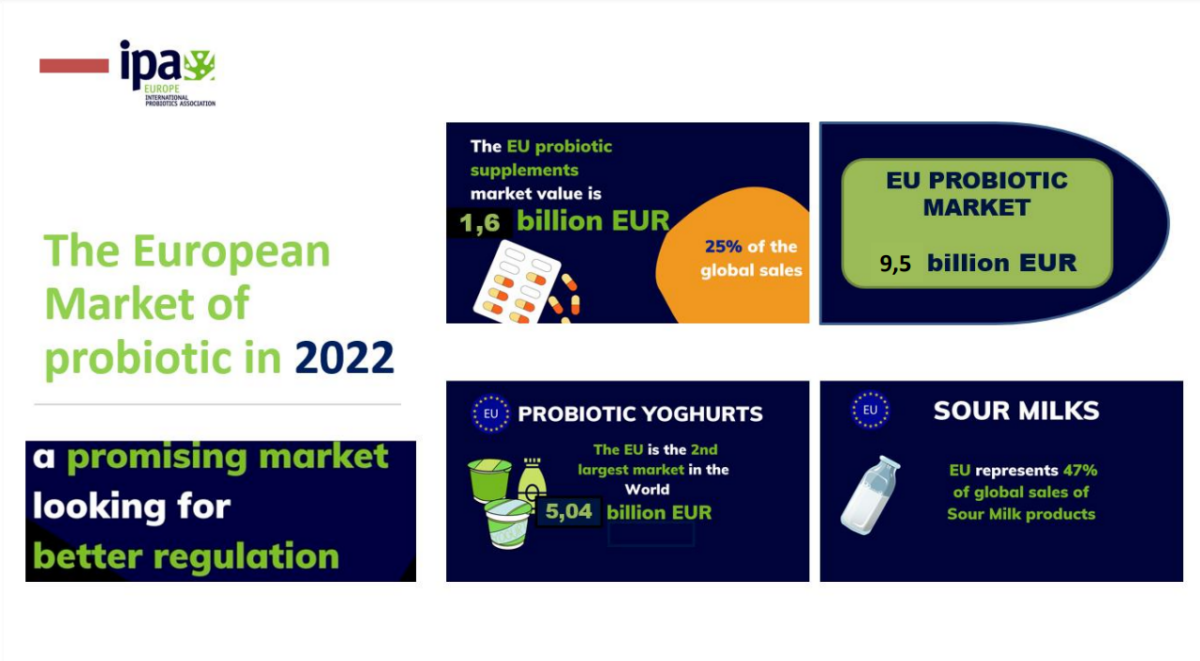
9.5 billion of probiotic sales in Europe, and billions of consumers looking for probiotics.
“How can better regulation strengthen the knowledge of probiotics for consumer health?” The virtual conference on 24th April 2023 featured an opening debate on the science of probiotics, the lack of regulation and how greater clarity can benefit the European industry while providing better information and transparency to consumers. PRESS-RELEASE ...
IPA Probiota World Congress in Barcelona on the 8th of February 2023
Speaking at IPA Probiota World Congress in Barcelona on 8 February 2023, Rosanna Pecere, Executive Director of IPA Europe, presented the overview of the European market ‘It is not only “in some third countries” that the use of the term ‘probiotics’ may be regulated differently’ she said. Since 2018, some EU ...
IPA Europe and EDA joint position on probiotics
What are probiotic microorganisms?
Probiotics are live microorganisms that can be formulated into many different types of products, including foods, drugs, and dietary supplements.
Species of Lactobacillus and Bifidobacterium are most commonly used as probiotics, but the yeast Saccharomyces cerevisiae and Bacillus species are also used as probiotics. Lactic acid bacteria (LAB), including species of Lactobacillus, which have been used for preservation of food by fermentation for thousands of years, can serve a dual function by acting as agents of food fermentation and, in addition, potentially imparting beneficial effects.
However, the term “probiotic” should be reserved for live microorganisms that have been shown in controlled human studies to accomplish their beneficial action. Fermentation of food provides characteristic taste profiles and lowers the pH, which can help in prevention of spoilage microorganisms and potential pathogens.
discover more